ames test results interpretations|Ames Test: Principle, Procedure, Result Interpretation, Applications : Cebu The Ames test is a widely accepted bacterial assay to detect the mutagenicity in pathogenic bacteria. In this protocol, although we have shown the step wise methodology to perform Ames assay applicable for three strains, this method can be used for studying . Fibisco. Biscuits Foods Grocery Snacks Mura: Pe bags, cafes, candy store & more. Eng Bee Tin Official Store Hopia: Fisco tin is a brand of biscuits making it the ideal choice for restaurants, cafes. Rebisco Biscuit Buy 1 Take 1: Give it, or use it as a gift for family and friends. Marie Time Biscuit for Baby: Marie biscuit is the best choice for baby food. .
PH0 · The test that changed the world: The Ames test and the
PH1 · Progress in Predicting Ames Test Outcomes from Chemical
PH2 · Microbial Mutagenicity Assay: Ames Test
PH3 · Microbial Mutagenicity Assay: Ames Test
PH4 · Ames test
PH5 · Ames Test: Principle, Procedure, Result Interpretation, Applications
PH6 · Ames Test: Principle, Procedure, Result Interpretation,
PH7 · Ames Test – Introduction, Principle, Procedure, Uses
PH8 · Ames Test (Bacterial Reverse Mutation Test): Why, When, and
PH9 · Ames Test
PH10 · 3.4: Ames Test
Harry Potter Clue!! The secret passages change and doors can lock when you spin the wheels. First time playing it and it's a blast! Needed for every Harry Potter fan!!! . it's a lot quicker with more people once everybody got the rules down. The expansion on the other hand is very brutal. It took us around 20 tries to finish the first .
ames test results interpretations*******The Ames test is a widely accepted bacterial assay to detect the mutagenicity in pathogenic bacteria. In this protocol, although we have shown the step wise methodology to perform Ames assay applicable for three strains, this method can be used for studying .
Ames Test: Principle, Procedure, Result Interpretation, ApplicationsThe Microbial mutagenicity Ames test is a bacterial bioassay accomplished in vitro .ames test results interpretations Ames Test: Principle, Procedure, Result Interpretation, ApplicationsThe Microbial mutagenicity Ames test is a bacterial bioassay accomplished in vitro . Result Interpretation. The mutagenicity of chemicals is proportional to number of colonies observed. If there is a large .
The Ames test, developed by Bruce Ames (1928–) in the 1970s, is a method that uses bacteria for rapid, inexpensive screening of the carcinogenic potential of new chemical .The Salmonella typhimurium /mammalian microsome assay is the most widely used short-term test to identify genetic damage. This is used to assess the mutagenic and .
The Ames test is a widely employed method that uses bacteria to test whether a given chemical can cause mutations in the DNA of the test organism. More formally, it is a . Ames test. Mutagenicity. Genetic toxicity history. 1. Introduction. The Salmonella/mammalian-microsome mutagenicity test, widely known as the Ames Test, . The Ames test, a biological assay that utilizes bacterial strains such as Salmonella typhimurium, is a widely used method for assessing chemical mutagenicity .The Ames test is a bacterial gene mutation assay widely used for its simplicity, accuracy and low cost (OECD, 1997a ). The assay measures the number of colonies formed after .
Ames test is a bacterial assay based on backmutation of different histidine-requiring strains of Salmonella typhimurium (see Fig. A61 ). Reversions are capable of .
Pharmacology and toxicology of α- and β-Asarone: A review of preclinical evidence. Ranjithkumar Chellian, . Zahurin Mohamed, in Phytomedicine, 2017. Ames test. Ames test is the most widely used test to predict the mutagenicity of any compounds, and if the test results are positive, it indicates that the compound may be a carcinogen. In Ames .The Ames test is a commonly used method that utilizes bacteria to test whether a particular chemical can cause mutations in the DNA of the test organism. It is a biological assay that is formally used to assess the .The /mammalian microsome assay is the most widely used short-term test to identify genetic damage. This is used to assess the mutagenic and antimutagenic potential of compounds and mixtures. This assay uses histidine-dependent strains to detect mutations, e.g., substitutions, additions, or deletions of one or several DNA nucleotides reverting .U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) June 2012 ICH. Guidance .
Ames test investigates the potential of the test compound to result in a back mutation that causes the gene to regain its function and grow in a histidine-free medium. Cyprotex offers the Ames test as an early stage assessment of genotoxicity using a miniaturised screening version which requires less compound and evaluates two of the most common . The bacterial reverse mutation test developed by Bruce Ames and his colleagues [1, 2] is perhaps the most widely used short-term bioassay to identify genetic damage that leads to gene mutation.This is a simple tool that can be used to detect the mutagenic and antimutagenic potential of environmental chemicals, environmental . The bacterial reverse mutation test, known as the Ames test, is often used to identify and characterize the mutagenicity of chemicals in basic research, and to examine the safety of industrial products prior to approval by regulatory agencies [1,2,3,4,5,6].The structural alerts for mutagenicity derived from the results are also used by regulatory .
The Ames Test is a widely accepted and simple test that can be used to determine the mutagenic and antimutagenic potential of various chemicals, pesticides and hormones, foods, drugs, and so on .
Interpretation of Ames Test Data: Guidelines. The criteria in determining a positive result from the Ames test include a reproducible increase in the number of revertants or a dose-related increase in mutant number. A positive result in the Ames test will usually initiate other mutagenicity assays including those with mammalian cells. If the .
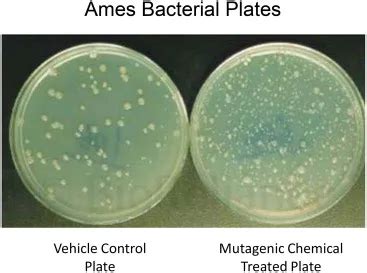
The OECD Test Guideline (TG) 471 describes the conduct of the standard agar plate Ames assay, re. Bacterial mutagenicity assays: Vehicle and positive control results from the standard Ames assay, the 6‐ and 24‐well miniaturized plate incorporation assays and the Ames II™ assay - Pant - 2016 - Environmental and Molecular .Interpreting the results of the Ames test involves analyzing the colony growth on the selective media and comparing it to the control plates. Here is a detailed explanation of how to interpret the Ames test results: Colony Counting: After the plates have been incubated, the colonies that have grown on the selective media are counted. . In genotoxicity tests using mammalian cells, a DMSO concentration of 1 % is often used. In this chapter, a detailed protocol for this slightly modified preincubation assay is presented, together with data useful for the performance of the Ames test and the interpretation of the results. Testing your heart health: These blood tests check for immediate heart problems and provide information about your risk of developing heart disease. Interpreting A1C: The hemoglobin A1C test .

RT3 tests can detect euthyroid sick syndrome (ESS), a condition in which abnormal thyroid hormone levels are due to an illness unrelated to the thyroid gland.; Tg tests can be used to predict long-term treatment outcomes.Research has shown that 4% of people with a Tg level under 1 will experience cancer recurrence within five years.; .
ames test results interpretations RT3 tests can detect euthyroid sick syndrome (ESS), a condition in which abnormal thyroid hormone levels are due to an illness unrelated to the thyroid gland.; Tg tests can be used to predict long-term treatment outcomes.Research has shown that 4% of people with a Tg level under 1 will experience cancer recurrence within five years.; . evaluate the current criteria for a valid Ames test and to provide recommendations for interpretation of test results. Currently, determination of a positive vs. a negative result is made by .
What is the main advantage of the Ames test for mutation detection? The Ames test has several key advantages: It is an easy and inexpensive bacterial assay for determining the mutagenicity of any chemical. Results are robust, and the Ames test can detect suitable mutants in large populations of bacteria with high sensitivity. The Ames test is an in vitro bacterial bioassay for identifying substances that cause gene mutations. Several compounds and drugs are mutagens or carcinogenic. The Ames test is used to investigate . A blood test – sometimes referred to as a blood panel – is a laboratory examination of a blood sample used to check for a variety of things, including the functioning of certain organs (such as the liver, kidneys, thyroid and heart), infections and certain genetic disorders, as well as to assess an individual’s general health.1. After the .
XNXX.COM 'milfs' Search, free sex videos. This menu's updates are based on your activity. The data is only saved locally (on your computer) and never transferred to us.
ames test results interpretations|Ames Test: Principle, Procedure, Result Interpretation, Applications